Main Goal: Towards sustainable crop protection strategy
The main goal of my group is to understand how plants interact with other organisms. We plan to systematically identify and characterize genes, proteins and small molecular compounds that are important for the interactions, by using genomics, proteomics and metabolomics approaches. We aim to elucidate signaling network system for plant immunity by analyzing expression patterns of defense related genes and their post transcriptional regulation, such as protein modification. Molecules in organisms interacting with plants, such as microbes, nematodes, parasitic plants will be investigated to understand how these organisms overcome plant immunity system and manipulate the host. Pathogen infection of plants is economically damaging to agricultural industries worldwide. Understanding the molecular basis of how plants interact with others in nature will provide new strategies to combat pathogens.
RIKEN Research 2019-09
Chemical Biology of Plant Immunity Signaling
Latest Papers:
Ishihama, N., Choi, S-W., Noutoshi, Y., Saska, I., Asai, S., Takizawa, K., He., S.Y., Osada, H., Shirasu, K. Oxicam-type nonsteroidal anti-inflammatory drugs inhibit NPR1-mediated salicylic acid pathway. (2021) Nature Comm 12: 7303. doi: 10.1038/s41467-021-27489-w
Noutoshi, Y., Okazaki, M., Kida, T., Nishina, Y., Morishita, Y., Ogawa, T., Suzuki, S., Shibata, D., Jikumaru, Y., Hanada, A., Kamiya, Y., and Shirasu, K. Novel plant immune-priming compounds identified via high-throughput chemical screening target salicylic acid glucosyltransferases in Arabidopsis (2012) Plant Cell 24:3795-3804.
Noutoshi, Y., Jikumaru, Y., Kamiya, Y., and Shirasu, K. ImprimatinC1, a novel plant immune-priming compound, functions as a partial agonist of salicylic acid. (2012) Scientific Reports 2:705
Noutoshi, Y., Ikeda, M., and Shirasu, K. Diuretics prime plant immunity in Arabidopsis thaliana. (2012) PLOS One 7(10): e48443.
Noutoshi, Y., Ikeda, M., Saito, T., Osada, H., Shirasu, K. Sulfonamides identified as plant immune-priming compounds in high-throughput chemical screening increase disease resistance in Arabidopsis thaliana (2012) Front. Plant Sci. 3:245
Noutoshi, Y., Okazaki, M., and Shirasu, K. Isolation and characterization of plant immune-priming compounds ImprimatinB3 and-B4, potentiators of disease resistance response in Arabidopsis thaliana (2012) Plant Sig & Behavior 7:1526-1528.
Noutoshi, Y., Okazaki, M., and Shirasu, K. ImparimatinA and B, novel plant activators targeting metabolism of salicylic acid in Arabidopsis thaliana (2012) Plant Sig & Behavior 7:1715-1717.
Resistance against Colletotrichum fungi
gloeosporioides and identified their effector candidates.
Related papers:
Gan, P., Hiroyama, R., Tsushima, A., Masuda, S., Shibata, A., Ueno, A., Kumakura, N., Narusaka, M., Hoat, T. X., Narusaka, Y., Takano, Y., Shirasu, K. Subtelomeric regions and a repeat-rich chromosome harbor multicopy effector gene clusters with variable conservation in multiple plant pathogenic Colletotrichum species. (2021) Env Microbiol. doi: 10.1111/1462-2920.15490
Tsushima, A., Gan, P., Kumakura, N., Narusaka, M., Takano, Y., Narusaka, Y., Shirasu, K. Genomic plasticity mediated by transposable elements in the plant pathogenic fungus Colletotrichum higginsianum (2019) Genome Biology and Evolution, evz087
Gan P., Tsushima A., Hiroyama R., Narusaka M., Takano, Y., Narusaka Y., Kawaradani M., Damm U. and Shirasu K. Colletotrichum shisoi sp. nov., an anthracnose pathogen of Perilla frutescens in Japan: molecular phylogenetic, morphological and genomic evidence. (2019) Sci. Rep. 9:13349.
Kumakura, N., Ueno, A., Shirasu, K. (2018) Establishment of a selection marker recycling system for sequential transformation of the plant pathogenic fungus Colletotrichum orbiculare. Molecular Plant Pathology.20: 447-459. doi: 10.1111/mpp.12766
Gao, L., Narita, K., Kumakura, N., Gan, P., Minami, A., Ozaki, T., Liu, C., Lei, X., Shirasu, K. Oikawa, H. Identification of novel sesterterpenes by the genome mining of phytopathogenic fungi Phoma and Colletotrichum sp. (2018) Tetrahedron Letters. in press.
Azmi, N.S.A. Singkaravanit-Ogawa, S., Ikeda, K., Kitakura, S., Inoue, Y., Narusaka, Y., Shirasu, K., Kaido, M., Mise, K., and Takano, Y. Inappropriate expression of an NLP effector in Colletotrichum orbiculare impairs infection on Cucurbitaceae cultivars via plant recognition of the C-terminal region (2018) Mol. Plant Micro. Int. 31: 101-111.
Gan, P., Narusaka, M., Tsushima, A., Narusaka, Y., Takano, Y., Shirasu, K. Draft genome assembly of Colletotrichum chlorophyti, a pathogen of herbaceous plants (2017) Genome Announcement 5:e01733-16
Gan, P., Nakata, N., Suzuki, T., Shirasu, K. Markers to distinguish different species of anthracnose fungi identify Colletotrichum fructicola as the predominant virulent species in strawberry plants in the Chiba prefecture of Japan (2017) J. Gen. Plant Path. 83: 14-22.
Gan, P., Narusaka, M., Kumakura, N., Tsushima, A., Takano, Y., Narusaka, Y., Shirasu, K. Genus-wide comparative genome analyses of Colletotrichum species reveal specific gene family losses and gains during adaptation to specific infection lifestyles. (2016) Genome Biology and Evolution. 8: 1467-1481.
Narusaka, M., Toyoda, K., Shiraishi, T., Iuchi, S., Takano, Y., Shirasu, K., Narusaka, Y. Leucine zipper motif in RRS1 is crucial for the regulation of Arabidopsis dual resistance protein complex RPS4/RRS1 (2015) Sci Rep. 6: 18702.
Crouch, J., O’Connell, R., Gan, P., Buiate, E., Torres, M.F., Beirn, L., Shirasu, K., Vaillancourt, L. The Genomics of Colletotrichum. (2014) in R. A. Dean et al. (eds.), Genomics of Plant-Associated Fungi: Monocot Pathogens, Springer-Verlag Berlin Heidelberg, 69-102.
Narusaka, M., Hatakeyama, K., Shirasu, K., and Narusaka, Y., Arabidopsis dual resistance proteins, both RPS4 and RRS1, are required for resistance to bacterial wilt in transgenic Brassica crops (2014) Plant Sig & Behavior. 9: e29130
Gan, P., Ikeda, K., Irieda, H., Narusaka, M., O’Connell, R.J., Narusaka, Y., Takano, Y., Kubo, Y., Shirasu, K. Comparative genomic and transcriptomic analyses reveal the hemibiotrophic stage shift of Colletotrichum fungi. (2013) New Phytologist 197: 1236-1249.
O'Connell R.J. et. al., Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses (2012) Nature Genetics in press
(above two papers are highlighted by
McDowell J. Genomic and transcriptomic insights into lifestyle transitions of a hemi-biotrophic fungal pathogen (2013))
Narusaka, M., Kubo, Y., Hatakeyama, K., Imamura, J., Ezura, H., Nanasato, Y., Tabei, Y., Takano, Y., Shirasu, K., and Narusaka, Y.. Interfamily transfer of dual NB-LRR genes confers resistance to multiple pathogens. (2013) PLOS ONE 8: e55954
(this paper is highlighted by Mukhtar, S.M., Engineering NLR immune receptors for broad-spectrum disease resistance (2013) Trends Plant Sci)
Hiruma, K., Fukunaga, S., Bednarek, P., Piślewska-Bednarek, M., Watababe, S., Narusaka, Y., Shirasu, K., and Takano, Y. Glutathione and tryptophan metabolism are required for Arabidopsis immunity during the hypersensitive response to hemibiotrophs. (2013) Proc. Natl. Acad. Sci. USA. 110: 9589-9594.
Narusaka, M., Ohtani, M., Demura, T., Shimada, R., Shirasu, K., Narusaka, Y. Development of a model system comprising Populus as a model tree and Colletotrichum species as a model pathogen for studying host–pathogen interaction (2012) Plant Biotechnology in press.
Narusaka M., Shirasu K., Noutoshi Y., Kubo Y., Shiraishi T., Iwabuchi M. and Narusaka Y. RRS1 and RPS4 provide a dual Resistance-gene system against fungal and bacterial pathogens. (2009) Plant J. 60:218-226.
Miya, A., Albert, P., Shinya, T., Desaki, Y., Ichimura, K., Shirasu, K., Narusaka, Y., Kawakami, N., Kaku, H and Shibuya, N. CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. (2007) Proc. Natl. Acad. Sci. 104:19613-19618.
Characterization of resistance signaling complxes
Latest papers:
Kadota, Y., Shirasu, K. and Zipfel, C. Regulation of the NADPH oxidase RBOHD during plant immunity. (2015) Plant Cell Physiol..56: 1472-1480
Kadota, Y., Jan Sklenar, J., Derbyshire, P., Stransfeld, L., Asai, S., Ntoukakis, V., Jones, J.D.G., Shirasu, K., Menke, F., Jones, A., and Zipfel, C. Direct regulation of the NADPH oxidase RBOHD by the PRRassociated kinase BIK1 is required for ROS bu and plant immunity. (2014) Mol. Cell 54: 43-55.
Kadota, Y. and Shirasu, K. The HSP90 complex of plants (2012) Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1823: 689–697.
Zhang M.*, Kadota Y.*, Prodromou C., Shirasu K.#, Pearl L. H.# Structural Basis for Assembly of Hsp90-Sgt1-CHORD Protein Complexes: Implications for Chaperoning of NLR Innate Immunity Receptors. (2010) Molecular Cell 39:269-281.
*These authors contributed equally to this work.
Kadota Y., Shirasu K. and Guerois R. NLR sensors meet at the SGT1HSP90 crossroad. (2010) Trends in Biochem. Sci. 35:199-207.
Shirasu, K. The HSP90-SGT1 Chaperone Complex for NLR Immune Sensors. (2009) Annual Review of Plant Biology 60:139-164
Kadota, Y., Amigues, B., Ducassou, L., Madaoui, H., Ochsenbein, F., Guerois, R. and Shirasu, K. Structural and functional analysis of SGT1-HSP90 core complex required for innate immunity in plants. (2008) EMBO reports 9:1209-1215.
Zhang, M., Boter, M., Li, K., Kadota, Y., Panaretou, B., Prodromou, C., Shirasu, K. and Pearl, L. Structural and functional coupling of Hsp90- and Sgt1-centred multi-protein complexes. (2008) EMBO J 27:2789-2798.
Boter, M., Amigues, B., Peart, J., Breuer, C., Kadota, Y., Casais, C., Moore, G., Kleanthous, C., Ochsenbein, F., Shirasu, K. and Guerois, R. Structural and functional analysis of SGT1 reveals that its interaction with HSP90 is required for the accumulation of Rx, an R protein involved in plant immunity. (2007) Plant Cell 19(11):3791-3804.
Characterization of kinases and ubiquitin ligases involved in plant immunity
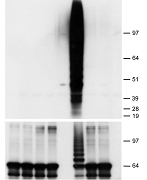
Related papers:
Kato, H., Nemoto, K., Shimizu, M., Abe, A., Asai, S., Ishihama, N., S. Matsuoka, S., Daimon, T., Ojika, M., Kawakita, K., Onai, K., Shirasu, K., M. Yoshida, M., Ishiura, M., Takemoto, D., Takano, Y., R. Terauchi, R. Recognition of pathogen-derived sphingolipids in Arabidopsis. (2022) Science. 376: 857-860. doi: 10.1126/science.abn0650.
Laohavisit A, Wakatake T, Ishihama N, Mulvey H, Takizawa K, Suzuki T, Shirasu K. Quinone perception in plants via leucine-rich repeat receptor-like kinases. (2020) Nature 587: 92-97. doi: 10.1038/s41586-020-2655-4
Kadota, Y. , Liebrand, T. W., Goto, Y. , Sklenar, J. , Derbyshire, P. , Menke, F. L., Torres, M. , Molina, A. , Zipfel, C. , Coaker, G. and Shirasu, K. (2018), Quantitative phosphoproteomic analysis reveals common regulatory mechanisms between effector‐ and PAMP‐triggered immunity in plants. New Phytol. doi: 10.1111/nph.15523
Stegmann, M., Anderson, R.G., Ichimura, K., Pecenkova, T., Reuter, P., Zarsky, V., McDowell, J.M., Shirasu, K., and Trujillo, M. The Ubiquitin Ligase PUB22 Targets a Subunit of the Exocyst Complex Required for PAMP-Triggered Responses in Arabidopsis. (2012) Plant Cell 24:4703-4716.
Nakagami H., Sugiyama N., Mochida K., Daudi A., Yoshida Y., Toyoda T., Tomita M., Ishihama Y. and Shirasu K. Large-scale comparative phosphoproteomics identifies conserved phosphorylation sites in plants. (2010) Plant Physiol. 153:1161-1174.
Trujillo, M., Ichimura, K., Casais, C. and Shirasu, K. Negative regulation of PAMP-triggered immunity by E3 ubiquitin ligase triplet in Arabidopsis. (2008) Current Biology 18:1396-1401.
Sugiyama, N., Nakagami, H., Mochida, K., Daudi, A., Tomita, M., Shirasu, K. and Ishihama, Y. Large-scale phosphorylation mapping reveales the extent of tyrosine phosphorylation in Arabidopsis. (2008) Molecular Systems Biology 4:193
Ichimura, K., Casais, C., Peck, S.C., Shinozaki, K. and Shirasu, K.
MEKK1 is required for MPK4 activation and regulates tissue specific and temperature dependent cell death in Arabidopsis.
J. Biol. Chem. (2006) 281:36969-36976.
Molecular studies on parasitic plants

Latest papers:
Ogawa, S., Cui, S., White, A. R. F., Nelson, D.C., Yoshida, Y., Shirasu, K. Strigolactones are chemoattractants for host tropism in Orobanchaceae parasitic plants. (2022) Nature Communications 13: 4653. doi: 10.1038/s41467-022-32314-z
Ogawa, S., Wakatake, T., Spallek, T., Ishida, J. K., Sano, R., Kurata, T., Demura, T., Yoshida, S., Ichihashi, Y., Schaller, A., Shirasu, K. Subtilase activity in the intrusive cells mediates haustorium maturation in parasitic plants. (2021) Plant Physiol. 185: 1381-1394. doi: 10.1093/plphys/kiab001
Mutuku, J.M., Cui, S., Yoshida, S., Shirasu, K. Orobanchaceae parasite-host interactions. (2021) New Phytologist. 230:46-59. doi: 10.1111/nph.17083.
Fishman, M.R. and Shirasu, K. How to resist parasitic plants: pre- and post attachment strategies. (2021) Curr. Opin. Plant Biol. 62:102004. doi: 10.1016/j.pbi.2021.102004
Cui, S., Kubota, T., Nishiyama, T., Ishida, J.K., Shigenobu, S., Shibata, T. F., Toyoda, A., Hasebe, M., Shirasu, K., Yoshida, S. Ethylene signaling mediates host invasion by parasitic plants. (2020) Sci. Adv. 6: eabc2385. doi: 10.1126/sciadv.abc2385
Kurotani, K.-I., Wakatake, T., Ichihashi, Y., Okayasu, K., Sawai, Y., Ogawa, S., Suzuki, T., Shirasu, K., Notaguchi, M. Host-parasite tissue adhesion by a secreted type of β-1,4-glucanase in the parasitic plant Phtheirospermum japonicum. (2020) Comm Biol. 3: 407. doi: 10.1038/s42003-020-01143-5
Wakatake T, Ogawa S, Yoshida S, Shirasu K. Auxin transport network underlies xylem bridge formation between the hemi-parasitic plant Phtheirospermum japonicum and host Arabidopsis. (2020) Development. dev.187781 doi: 10.1242/dev.187781.
Yoshida et al. Genome sequence of Striga asiatica provides insight into the evolution of plant parasitism. (2019) Curr. Biol. 29: 3041-3052.E4. doi: 10.1016/j.cub.2019.07.086
Wakatake, T., Yoshida, S., Shirasu, K. Induced cell fate transitions at multiple cell layers configure haustorium development in parasitic plants (2018) Development. in press.
Ichihashi, Y., Kusano, M., Kobayashi, M., Suetsugu, K., Yoshida, S., Wakatake, T., Kumaishi, K., Shibata, A., Saito, K., and Shirasu, K. (2018) Transcriptomic and metabolomic reprogramming from roots to haustoria in the parasitic plant, Thesium chinense. (2018) Plant Cell Physiol 59:724-733.
Spallek, T., Melnyk, C.W., Wakatake, T., Zhang, J., Sakamoto, Y., Kiba, T., Yoshida, S., Matsunaga, S., Sakakibara, H., Shirasu, K. Inter-species hormonal control of host root morphology by parasitic plants. (2017) Proc. Natl. Acad. Sci. USA. 114:5283-5288.
Ishida, J. K., Wakatake, T., Yoshida, S., Takebayashi, Y., Kasahara, H., Wafula, E., dePamphilis, C. W., Shigetou N., Shirasu, K. Local auxin biosynthesis mediated by a YUCCA flavin monooxygenase regulates the haustorium development in the parasitic plant Phtheirospermum japonicum. (2016) Plant Cell 28:1795-1814.
Yoshida, S., Cui, S., Ichihashi, Y., Shirasu, K. The haustorium, a specialized invasive organ in parasitic plants. (2016) Annu Rev Plant Biol. 67:643-667.
Cui, S., Wakatake, T., Hashimoto, K., Saucet, S.B., Toyooka, K., Yoshida, S. and Shirasu, K. Haustorial hairs are specialized root hairs that support parasitism in the facultative parasitic plant, Phtheirospermum japonicum (2016) Plant Physiol.170: 1492-1503.
Conn, C. E., Bythell-Douglas, R., Neumann, D., Yoshida, S., Whittington, B., Westwood, J. H., Shirasu, K., Bond, C. S. Dyer, K. A., Nelson, D. C. (2015) Convergent evolution of strigolactone perception enabled host detection in parasitic plants. Science 349: 540-543.
Mutuku, J.M., Yoshida, S., Shimizu, T., Ichihashi, Y., Wakatake., T., Seo, 168:M., Takahashi., A., Shirasu, K. The WRKY45-dependent signaling pathway is required for resistance against Striga parasitism. (2015) Plant Physiol 168:1152-1163.
Ichihashi, Y., Mutuku, J.M., Yoshida, S., and Shirasu, K. (2015) Transcriptomics exposes the uniqueness of parasitic plants. Briefings in Functional Genomics. in press.,
Spallek, T., Mutuku, M., and Shirasu, K. The genus Striga: a witch profile. (2013) Mol Plant Path in press
Yoshida, S. and Shirasu, K. Plants that attack plants: molecular elucidation of plant parasitism. (2012) Curr Opin Plant Biol 15:708-713
Yoshida S., Maruyama S., Nozaki H., Shirasu K. Horizontal Gene Transfer by the Parasitic Plant Striga hermonthica. (2010) Science 328:1128
Yoshida S., Ishida J. K., Kamal N. M., Ali A. M., Namba S. and Shirasu K. A full-length enriched cDNA library and expressed sequence tag analysis of the parasitic weed, Striga hermonthica. (2010) BMC Plant Biol. 10:55
Yoshida, S. and Shirasu, K. Multiple layers of incompatibility to the parasitic witchweed, Striga hermonthica. (2009) New Phytologist
Umehara, M., Hanada, A., Yoshida, S., Akiyama, K., Arite, T., Takeda-Kamiya, N., Magome, H., Kamiya, Y., Shirasu, K., Yoneyama, K., Kyozuka, J. and Yamaguchi, S. Inhibition of shoot branching by new terpenoid plant hormones. (2008) Nature 455(7210):195-200.
De novo genome sequencing of plant-assoicated organisms
Eucaryotes
- Parasitic plants
- Striga asiatica
- Ptheirospermum japonicum
- Nematodes
- Fungi
- Colletotrichum
- C. orbiculare 104-T, MAFF24022, NCBI
- C. fructicola Nara Gc5, NCBI
- C. incanum, MAFF238712, NCBI
- C. chlorophyti NTL11, NCBI
- C. fructicola NTL1-1b
- C. aenigma Cg56
- C. siamense Cg363
- C. fructicola 413
- C. fructicola 415
- C. fructicola 245
- C. fructicola S4
- C. siamense CAD1
- C. siamense CAD2
- C. siamense CAD4
- C. siamense CAD5
- C. musae CGW01
- C. tropicale S9275
- C. shisoi new species
- Fusarium
Procaryotes
- Pseudomonas
- P. amygdali pv. tabaci strain 6605


