持続的な耐病性植物の作出をめざして
植物は本来病原体に対する高い防御能力を備えています。本研究グループはこの植物の耐病性に関与する遺伝子、タンパク質、および低分子化学物質をゲノミクス、プロテオミクス、メタボロミクス的解析手法を用いて網羅的に単離し、植物の免疫システムの解明をめざします。感染応答遺伝子の発現パターンと信号伝達ネットワークを解析し、また免疫システムの制御に関与するタンパク質の修飾などによるスイッチ機能を明らかにし、持続的な耐病性植物の作出をめざします。
ケミカルバイオロジーによる植物免疫研究
最新論文:
Ishihama, N., Choi, S-W., Noutoshi, Y., Saska, I., Asai, S., Takizawa, K., He., S.Y., Osada, H., Shirasu, K. Oxicam-type nonsteroidal anti-inflammatory drugs inhibit NPR1-mediated salicylic acid pathway. (2021) Nature Comm. 12: 7303. doi: 10.1038/s41467-021-27489-w doi: 10.1101/2020.09.25.311100.
Noutoshi, Y., Okazaki, M., Kida, T., Nishina, Y., Morishita, Y., Ogawa, T., Suzuki, S., Shibata, D., Jikumaru, Y., Hanada, A., Kamiya, Y., and Shirasu, K. Novel plant immune-priming compounds identified via high-throughput chemical screening target salicylic acid glucosyltransferases in Arabidopsis (2012) Plant Cell 24:3795-3804.
Noutoshi, Y., Jikumaru, Y., Kamiya, Y., and Shirasu, K. ImprimatinC1, a novel plant immune-priming compound, functions as a partial agonist of salicylic acid. (2012) Scientific Reports 2:705
Noutoshi, Y., Ikeda, M., and Shirasu, K. Diuretics prime plant immunity in Arabidopsis thaliana. (2012) PLOS One 7(10): e48443.
Noutoshi, Y., Ikeda, M., Saito, T., Osada, H., Shirasu, K. Sulfonamides identified as plant immune-priming compounds in high-throughput chemical screening increase disease resistance in Arabidopsis thaliana (2012) Front. Plant Sci. 3:245
Noutoshi, Y., Okazaki, M., and Shirasu, K. Isolation and characterization of plant immune-priming compounds ImprimatinB3 and-B4, potentiators of disease resistance response in Arabidopsis thaliana (2012) Plant Sig & Behavior 7:1526-1528.
Noutoshi, Y., Okazaki, M., and Shirasu, K. ImparimatinA and B, novel plant activators targeting metabolism of salicylic acid in Arabidopsis thaliana (2012) Plant Sig & Behavior 7:1715-1717.
病原体の分泌エフェクター戦略を標的とする作物保護技術の基盤開発
これまでに
ウリ類炭疽病菌ゲノム
イチゴ炭疽病菌ゲノム
ダイコン炭疽病菌ゲノム
イネ科炭疽病菌ゲノム
アブラナ科炭疽病菌ゲノム
C. chlorophyti ゲノム
C. shisoi ゲノム
フザリウムゲノム
イチゴ炭疽病菌判別マーカー
フザリウム病原性マーカー
を発表しています。
共同研究者
京都大学大学院農学研究科 髙野義孝
岡山県農林水産総合センター生物科学研究所 鳴坂義弘
京都府大生命環境科学研究科 久保康之
北海道大学大学院理学研究科 及川英秋
東京農工大学 有江力
関連論文
Tsushima, A., Gan, P., Kumakura, N., Narusaka, M., Takano, Y., Narusaka, Y., Shirasu, K. Genomic plasticity mediated by transposable elements in the plant pathogenic fungus Colletotrichum higginsianum (2019) Genome Biology and Evolution, evz087
Gan P., Tsushima A., Hiroyama R., Narusaka M., Takano, Y., Narusaka Y., Kawaradani M., Damm U. and Shirasu K. Colletotrichum shisoi sp. nov., an anthracnose pathogen of Perilla frutescens in Japan: molecular phylogenetic, morphological and genomic evidence. (2019) Sci. Rep. 9:13349.
Gan, P., Tsushima, A., Narusaka, M., Narusaka, Y., Takano, Y., Kubo, Y., Shirasu, K. Genome sequence resources for four phytopathogenic fungi from the Colletotrichum orbiculare species complex (2019) MPMI on line
Kumakura, N., Ueno, A., Shirasu, K. (2018) Establishment of a selection marker recycling system for sequential transformation of the plant pathogenic fungus Colletotrichum orbiculare. Molecular Plant Pathology.20: 447-459. doi: 10.1111/mpp.12766
Gao, L., Narita, K., Kumakura, N., Gan, P., Minami, A., Ozaki, T., Liu, C., Lei, X., Shirasu, K. Oikawa, H. Identification of novel sesterterpenes by the genome mining of phytopathogenic fungi Phoma and Colletotrichum sp. (2018) Tetrahedron Letters. in press.
Azmi, N.S.A. Singkaravanit-Ogawa, S., Ikeda, K., Kitakura, S., Inoue, Y., Narusaka, Y., Shirasu, K., Kaido, M., Mise, K., and Takano, Y. Inappropriate expression of an NLP effector in Colletotrichum orbiculare impairs infection on Cucurbitaceae cultivars via plant recognition of the C-terminal region (2018) Mol. Plant Micro. Int. 31: 101-111.
Gan, P., Narusaka, M., Tsushima, A., Narusaka, Y., Takano, Y., Shirasu, K. Draft genome assembly of Colletotrichum chlorophyti, a pathogen of herbaceous plants (2017) Genome Announcement 5:e01733-16
Gan, P., Nakata, N., Suzuki, T., Shirasu, K. Markers to distinguish different species of anthracnose fungi identify Colletotrichum fructicola as the predominant virulent species in strawberry plants in the Chiba prefecture of Japan (2017) J. Gen. Plant Path. 83: 14-22.
Gan, P., Narusaka, M., Kumakura, N., Tsushima, A., Takano, Y., Narusaka, Y., Shirasu, K. Genus-wide comparative genome analyses of Colletotrichum species reveal specific gene family losses and gains during adaptation to specific infection lifestyles. (2016) Genome Biology and Evolution. 8: 1467-1481.
Narusaka, M., Toyoda, K., Shiraishi, T., Iuchi, S., Takano, Y., Shirasu, K., Narusaka, Y. Leucine zipper motif in RRS1 is crucial for the regulation of Arabidopsis dual resistance protein complex RPS4/RRS1 (2015) Sci Rep. 6: 18702.
Crouch, J., O’Connell, R., Gan, P., Buiate, E., Torres, M.F., Beirn, L., Shirasu, K., Vaillancourt, L. The Genomics of Colletotrichum. (2014) in R. A. Dean et al. (eds.), Genomics of Plant-Associated Fungi: Monocot Pathogens, Springer-Verlag Berlin Heidelberg, 69-102.
Narusaka, M., Hatakeyama, K., Shirasu, K., and Narusaka, Y., Arabidopsis dual resistance proteins, both RPS4 and RRS1, are required for resistance to bacterial wilt in transgenic Brassica crops (2014) Plant Sig & Behavior. 9: e29130
Gan, P., Ikeda, K., Irieda, H., Narusaka, M., O’Connell, R.J., Narusaka, Y., Takano, Y., Kubo, Y., Shirasu, K. Comparative genomic and transcriptomic analyses reveal the hemibiotrophic stage shift of Colletotrichum fungi. (2013) New Phytologist 197:1236-1249.
O'Connell R.J. et. al., Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses (2012) Nature Genetics 44:1060-1065.
Narusaka, M., Kubo, Y., Hatakeyama, K., Imamura, J., Ezura, H., Nanasato, Y., Tabei, Y., Takano, Y., Shirasu, K., and Narusaka, Y.. Interfamily transfer of dual NB-LRR genes confers resistance to multiple pathogens. (2013) PLOS ONE 8: e55954
Hiruma, K., Fukunaga, S., Bednarek, P., Piślewska-Bednarek, M., Watababe, S., Narusaka, Y., Shirasu, K., and Takano, Y. Glutathione and tryptophan metabolism are required for Arabidopsis immunity during the hypersensitive response to hemibiotrophs. (2013) Proc. Natl. Acad. Sci. USA. 110: 9589-9594.
Narusaka, M., Ohtani, M., Demura, T., Shimada, R., Shirasu, K., Narusaka, Y. Development of a model system comprising Populus as a model tree and Colletotrichum species as a model pathogen for studying host–pathogen interaction (2012) Plant Biotechnology 29: 511-514.
Yaeno, T., Li, H., Chaparro-Garcia, A., Schornack, S., Koshiba, S., Watanabe, S., Kigawa, T., Kamoun, S., and Shirasu, K. Phosphatidylinositol monophosphate-binding interface in the oomycete RXLR effector AVR3a is required for its stability in host cells to modulate plant immunity. (2011) Proc. Natl. Acad. Sci. USA. 108: 14682-14687. PDF
Narusaka M., Shirasu K., Noutoshi Y., Kubo Y., Shiraishi T., Iwabuchi M. and Narusaka Y. RRS1 and RPS4 provide a dual Resistance-gene system against fungal and bacterial pathogens. (2009) Plant J. 60:218-226.
Miya, A., Albert, P., Shinya, T., Desaki, Y., Ichimura, K., Shirasu, K., Narusaka, Y., Kawakami, N., Kaku, H and Shibuya, N. CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. (2007) Proc. Natl. Acad. Sci. 104:19613-19618.
寄生植物の分子生物学的研究

解説・インタビュー:
植物を襲う植物(RIKEN NEWS 2020 11月号)
寄生植物の不思議 in 植物科学のトビラ (2021.3.1)
関連論文:
Ogawa, S., Cui, S., White, A. R. F., Nelson, D.C., Yoshida, Y., Shirasu, K. Strigolactones are chemoattractants for host tropism in Orobanchaceae parasitic plants. (2022) Nature Communications 13: 4653. doi: 10.1038/s41467-022-32314-z
Laohavisit A, Wakatake T, Ishihama N, Mulvey H, Takizawa K, Suzuki T, Shirasu K. Quinone perception in plants via leucine-rich repeat receptor-like kinases. (2020) Nature 587: 92-97. doi: 10.1038/s41586-020-2655-4
Wakatake T, Ogawa S, Yoshida S, Shirasu K. Auxin transport network underlies xylem bridge formation between the hemi-parasitic plant Phtheirospermum japonicum and host Arabidopsis. (2020) Development. dev.187781 doi: 10.1242/dev.187781
Cui, S., Kubota, T., Nishiyama, T., Ishida, J.K., Shigenobu, S., Shibata, T. F., Toyoda, A., Hasebe, M., Shirasu, K., Yoshida, S. Ethylene signaling mediates host invasion by parasitic plants. (2020) Sci. Adv. 6: eabc2385. doi: 10.1126/sciadv.abc2385
Kurotani, K.-I., Wakatake, T., Ichihashi, Y., Okayasu, K., Sawai, Y., Ogawa, S., Suzuki, T., Shirasu, K., Notaguchi, M. Host-parasite tissue adhesion by a secreted type of β-1,4-glucanase in the parasitic plant Phtheirospermum japonicum. Comm Biol. 3: 407. doi: 10.1038/s42003-020-01143-5
Ichihashi Y, Hakoyama T, Iwase A, Shirasu K, Sugimoto K, Hayashi M. 2020. Common mechanisms of developmental reprogramming - lessons from regeneration, symbiosis and parasitism. Front Plant Sci. 11:1084. doi: 10.3389/fpls.2020.01084.
Yoshida et al. Genome sequence of Striga asiatica provides insight into the evolution of plant parasitism. (2019) Curr. Biol. 29: 3041-3052.E4.
Wakatake, T., Yoshida, S., Shirasu, K. Induced cell fate transitions at multiple cell layers configure haustorium development in parasitic plants (2018) Development. 145: dev164848
Ichihashi, Y., Kusano, M., Kobayashi, M., Suetsugu, K., Yoshida, S., Wakatake, T., Kumaishi, K., Shibata, A., Saito, K., and Shirasu, K. (2018) Transcriptomic and metabolomic reprogramming from roots to haustoria in the parasitic plant, Thesium chinense. (2018) Plant Cell Physiol 59:724-733.
Spallek, T., Melnyk, C.W., Wakatake, T., Zhang, J., Sakamoto, Y., Kiba, T., Yoshida, S., Matsunaga, S., Sakakibara, H., Shirasu, K. Inter-species hormonal control of host root morphology by parasitic plants. (2017) Proc. Natl. Acad. Sci. USA. 114:5283-5288.
Ishida, J. K., Wakatake, T., Yoshida, S., Takebayashi, Y., Kasahara, H., Wafula, E., dePamphilis, C. W., Shigetou N., Shirasu, K. Local auxin biosynthesis mediated by a YUCCA flavin monooxygenase regulates the haustorium development in the parasitic plant Phtheirospermum japonicum. (2016) Plant Cell 28:1795-1814.
若竹崇雅、吉田聡子、白須賢 (2016) 根寄生植物の寄生メカニズム─ ゲノム解読とモデル実験系の確立で農業被害の撲滅に道 生物の科学 遺伝 70 (4) 289-293.
Yoshida, S., Cui, S., Ichihashi, Y., Shirasu, K. The haustorium, a specialized invasive organ in parasitic plants. (2016) Annu Rev Plant Biol. 67:643-667.
Cui, S., Wakatake, T., Hashimoto, K., Saucet, S.B., Toyooka, K., Yoshida, S. and Shirasu, K. Haustorial hairs are specialized root hairs that support parasitism in the facultative parasitic plant, Phtheirospermum japonicum (2016) Plant Physiol. pp.01786.
Conn, C. E., Bythell-Douglas, R., Neumann, D., Yoshida, S., Whittington, B., Westwood, J. H., Shirasu, K., Bond, C. S. Dyer, K. A., Nelson, D. C. (2015) Convergent evolution of strigolactone perception enabled host detection in parasitic plants. Science 349: 540-543.
Mutuku, J.M., Yoshida, S., Shimizu, T., Ichihashi, Y., Wakatake., T., Seo, 168:M., Takahashi., A., Shirasu, K. The WRKY45-dependent signaling pathway is required for resistance against Striga parasitism. (2015) Plant Physiol 168:1152-1163.
Ichihashi, Y., Mutuku, J.M., Yoshida, S., and Shirasu, K. (2015) Transcriptomics exposes the uniqueness of parasitic plants. Briefings in Functional Genomics. in press.,
Spallek, T., Mutuku, M., and Shirasu, K. The genus Striga: a witch profile. (2013) Mol Plant Path in press
Yoshida, S. and Shirasu, K. Plants that attack plants: molecular elucidation of plant parasitism. (2012) Curr Opin Plant Biol 15:708-713
Yoshida S., Maruyama S., Nozaki H., Shirasu K. Horizontal Gene Transfer by the Parasitic Plant Striga hermonthica. (2010) Science 328:1128
Yoshida S., Ishida J. K., Kamal N. M., Ali A. M., Namba S. and Shirasu K. A full-length enriched cDNA library and expressed sequence tag analysis of the parasitic weed, Striga hermonthica. (2010) BMC Plant Biol. 10:55
Yoshida, S. and Shirasu, K. Multiple layers of incompatibility to the parasitic witchweed, Striga hermonthica. (2009) New Phytologist
Umehara, M., Hanada, A., Yoshida, S., Akiyama, K., Arite, T., Takeda-Kamiya, N., Magome, H., Kamiya, Y., Shirasu, K., Yoneyama, K., Kyozuka, J. and Yamaguchi, S. Inhibition of shoot branching by new terpenoid plant hormones. (2008) Nature 455(7210):195-200.
耐病性シグナル複合体の研究
本研究の一部は科学研究費補助金(若手研究(S)によって推進されました。 研究進捗評価結果は こちら。
関連論文:
Kadota, Y., Shirasu, K. and Zipfel, C. Regulation of the NADPH oxidase RBOHD during plant immunity. (2015) Plant Cell Physiol..56: 1472-1480
Kadota, Y., Jan Sklenar, J., Derbyshire, P., Stransfeld, L., Asai, S., Ntoukakis, V., Jones, J.D.G., Shirasu, K., Menke, F., Jones, A., and Zipfel, C. Direct regulation of the NADPH oxidase RBOHD by the PRR associated kinase BIK1 is required for ROS burst and plant immunity. (2014) Mol. Cell 54: 43-55.
Kadota, Y. and Shirasu, K. The HSP90 complex of plants (2012) Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1823: 689–697.
Kadota, Y., Jan Sklenar, J., Derbyshire, P., Stransfeld, L., Asai, S., Ntoukakis, V., Jones, J.D.G., Shirasu, K., Menke, F., Jones, A., and Zipfel, C. Direct regulation of the NADPH oxidase RBOHD by the PRRassociated kinase BIK1 is required for ROS bu and plant immunity. (2014) Mol. Cell 54: 43-55.
Zhang M.*, Kadota Y.*, Prodromou C., Shirasu K.#, Pearl L. H.# Structural Basis for Assembly of Hsp90-Sgt1-CHORD Protein Complexes: Implications for Chaperoning of NLR Innate Immunity Receptors. (2010) Molecular Cell 39:269-281.
*These authors contributed equally to this work.
Kadota Y., Shirasu K. and Guerois R. NLR sensors meet at the SGT1HSP90 crossroad. (2010) Trends in Biochem. Sci. 35:199-207.
Shirasu, K. The HSP90-SGT1 Chaperone Complex for NLR Immune Sensors. (2009) Annual Review of Plant Biology 60:139-164
Kadota, Y., Amigues, B., Ducassou, L., Madaoui, H., Ochsenbein, F., Guerois, R. and Shirasu, K. Structural and functional analysis of SGT1-HSP90 core complex required for innate immunity in plants. (2008) EMBO reports 9:1209-1215.
Zhang, M., Boter, M., Li, K., Kadota, Y., Panaretou, B., Prodromou, C., Shirasu, K. and Pearl, L. Structural and functional coupling of Hsp90- and Sgt1-centred multi-protein complexes. (2008) EMBO J 27:2789-2798.
Boter, M., Amigues, B., Peart, J., Breuer, C., Kadota, Y., Casais, C., Moore, G., Kleanthous, C., Ochsenbein, F., Shirasu, K. and Guerois, R. Structural and functional analysis of SGT1 reveals that its interaction with HSP90 is required for the accumulation of Rx, an R protein involved in plant immunity. (2007) Plant Cell 19(11):3791-3804.
和文総説:
門田康弘、能年義輝、白須賢
抵抗性蛋白質複合体
蛋白質核酸酵素 (2007) 5月号増刊 Vol.52 No.6 717-723.
植物免疫に関するリン酸化とユビキチン化の研究
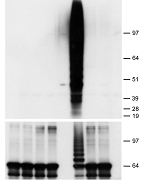
関連論文:
Kadota, Y. , Liebrand, T. W., Goto, Y. , Sklenar, J. , Derbyshire, P. , Menke, F. L., Torres, M. , Molina, A. , Zipfel, C. , Coaker, G. and Shirasu, K. Quantitative phosphoproteomic analysis reveals common regulatory mechanisms between effector‐ and PAMP‐triggered immunity in plants. (2019) New Phytol. 221:2160-2175.
Stegmann, M., Anderson, R.G., Ichimura, K., Pecenkova, T., Reuter, P., Zarsky, V., McDowell, J.M., Shirasu, K., and Trujillo, M. The Ubiquitin Ligase PUB22 Targets a Subunit of the Exocyst Complex Required for PAMP-Triggered Responses in Arabidopsis. (2012) Plant Cell 24:4703-4716.
Nakagami H., Sugiyama N., Mochida K., Daudi A., Yoshida Y., Toyoda T., Tomita M., Ishihama Y. and Shirasu K. Large-scale comparative phosphoproteomics identifies conserved phosphorylation sites in plants. (2010) Plant Physiol. 153:1161-1174.
Trujillo, M., Ichimura, K., Casais, C. and Shirasu, K. Negative regulation of PAMP-triggered immunity by E3 ubiquitin ligase triplet in Arabidopsis. (2008) Current Biology 18:1396-1401.
Sugiyama, N., Nakagami, H., Mochida, K., Daudi, A., Tomita, M., Shirasu, K. and Ishihama, Y. Large-scale phosphorylation mapping reveales the extent of tyrosine phosphorylation in Arabidopsis. (2008) Molecular Systems Biology 4:193
Maor, R., Jones, A., Nuhse, T.S., Studholme, D.J., Peck, S.C. and Shirasu, K. MudPIT analysis of ubiquitinated proteins in plants. Molecular & Cellular Proteomics (2007) 6(4):601-610.
Ichimura, K., Casais, C., Peck, S.C., Shinozaki, K. and Shirasu, K.
MEKK1 is required for MPK4 activation and regulates tissue specific and temperature dependent cell death in Arabidopsis. J. Biol. Chem. (2006) 281:36969-36976.
和文総説:
中神弘史、白須賢
ユビキチン結合領域を利用したユビキチン化タンパク質の精製法
バイオテクノロジージャーナル (2007) Vol.7 No.5 (9-10月号) 594-598.
市村和也、能年義輝、白須賢
植物免疫におけるMAPキナーゼカスケードの役割
化学と生物 (2006) Vol.44 No.7 466-471.
植物と相互作用する生物のゲノム解析
植物は数多くの生物と共に生きています。どうやって共に生きているのか?どのようにコミュニケーションをおこない、どのような物質を交換したり、あるいはお互いにどのような恩恵を受けているのでしょうか?また、その関係性はどのように進化してきたのか、そしてどのように進化するのか?それぞれその生物のゲノムを紐解くことによってその謎に迫ります。
関連論文
線虫
Meloidogyne arenaria
Sato, K., Kadota, Y., Gan, P., Bino, T., Uehara, T., Yamaguchi, K., Ichihashi, I., Maki, N., Iwahori, H., Suzuki, T., Shigenobu, S., Shirasu, K. High-Quality Genome Sequence of the Root-Knot Nematode Meloidogyne arenaria Genotype A2-O. (2018) Genome Announcement. 6: e00519-18. doi: 10.1128/genomeA.00519-18

